9+ Which Intervals Are Affected By The Addition Of A Catalyst
AH2O BNO2 CC2H6 DCO2 5According to Reference Table I which gas is formed from its elements as a result of an. Given the potential energy diagram for a chemical reaction.
Partial Incorporation Of La3 In Beta Zeolite For Isobutane 1 Butene Alkylation Springerlink
Suppose you add a catalyst to the system already at equilibrium nothing happens as the.

. Which expression represents the heat of reaction for a chemical change in terms of potential energy PE. Which intervals are affected by the addition of a fêtalyst. Which statement describes the energy of the particles in this sample during interval DE.
Which numbered interval will change with the addition of a catalyst to the system. Catalyst remains chemically unchanged at the end of a reaction. The catalyst would lower the hump or activation energy B also lowering the total energy A put into the reaction.
The different aspects of a reaction are. 1 Both potential energy and average kinetic energy increase. A catalyst is a compound or element that increases the rate of a chemical reaction eg.
Which intervals are affected by the. View the full answer. Which intervals are affected by the addition of a catalyst.
Which intervals are affected by the addition of a catalyst. The Effect of a Catalyst on Equilibrium. Which intervals are affected by the addition of a catalyst.
A catalyst will affect the rate of the forward reaction by changing the. Rate of reaction can be increased by using a catalyst. The reaction that produces ammonia is represented by balanced equation 2.
This also goes for exothermic processes. Which intervals are affected by the addition of a catalyst. At equilibrium the rate of forward reaction is equal to the rate of backward reaction.
How are the following aspects of a reaction affected by the addition of a catalyst. A catalyst lowers the activation energy for example by providing a surface for the reaction to occur on. How are the following aspects of a reaction affected by the addition of a catalyst.
The speed at which it occurs without itself being part of the reaction. The activation energy of the forward reaction. Adding a catalyst will decrease the activation energy both in the forward direction and the backward direction.
In the presence of a. 7 _ÿcŒOšzS 3 1 and 3 4 3 and 4 1 1 and 2 2 2 and 5. Intervals 1 and 3 are most affected by the addition of catalyst.
It means that the quantity and chemical composition of the catalyst remain unchanged at the end of the reaction. The activation energy of the backward reaction. Reactions can be sped up by the addition of a catalyst including reversible reactions involving a final equilibrium state.
When this happens the rate of the reaction will increase both in the forward. 1 1 and 2 2 1 and 3 3 2 and 4 4 3 and 4. Option 2 is correctWhat are catalystsCatalysts are those compounds that reduce the activation kghansah kghansah.
Aa catalyst Ban indicator Celectrical energy Dthermal energy 5The activation energy of a chemical reaction can be.

Chapter 18 Reaction Rates Equilibrium Flashcards Quizlet
11 3 Sitekonfiguration Mahara 20 04 Manual

Cisco Crosswork Situation Manager 8 0 X Implementer Guide Cisco

Progress In The Direct Catalytic Conversion Of Methane To Fuels And Chemicals Sciencedirect

Catalysts Free Full Text Selective Formation Of Para Xylene By Methanol Aromatization Over Phosphorous Modified Zsm 5 Zeolites Html
Effect Of The Catalytic System And Operating Conditions On Btx Formation Using Tetralin As A Model Molecule Springerlink

App Store Connect Help
Cnx Stats C02 M04 020 Jpg

Iii International Conference

Supramolecular Catalysis Of Acyl Transfer Within Zinc Porphyrin Based Metal Organic Cages Inorganic Chemistry
Catalysts Free Full Text Selective Formation Of Para Xylene By Methanol Aromatization Over Phosphorous Modified Zsm 5 Zeolites Html
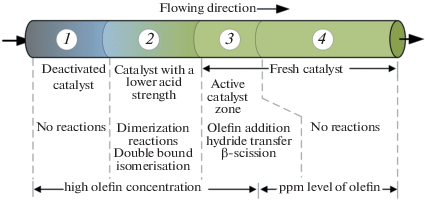
Comparison Of Isobutane N Butenes Alkylation Over Y Zeolite Catalyst In Cstr Fixed Bed And Circulating Flow Reactors Springerlink
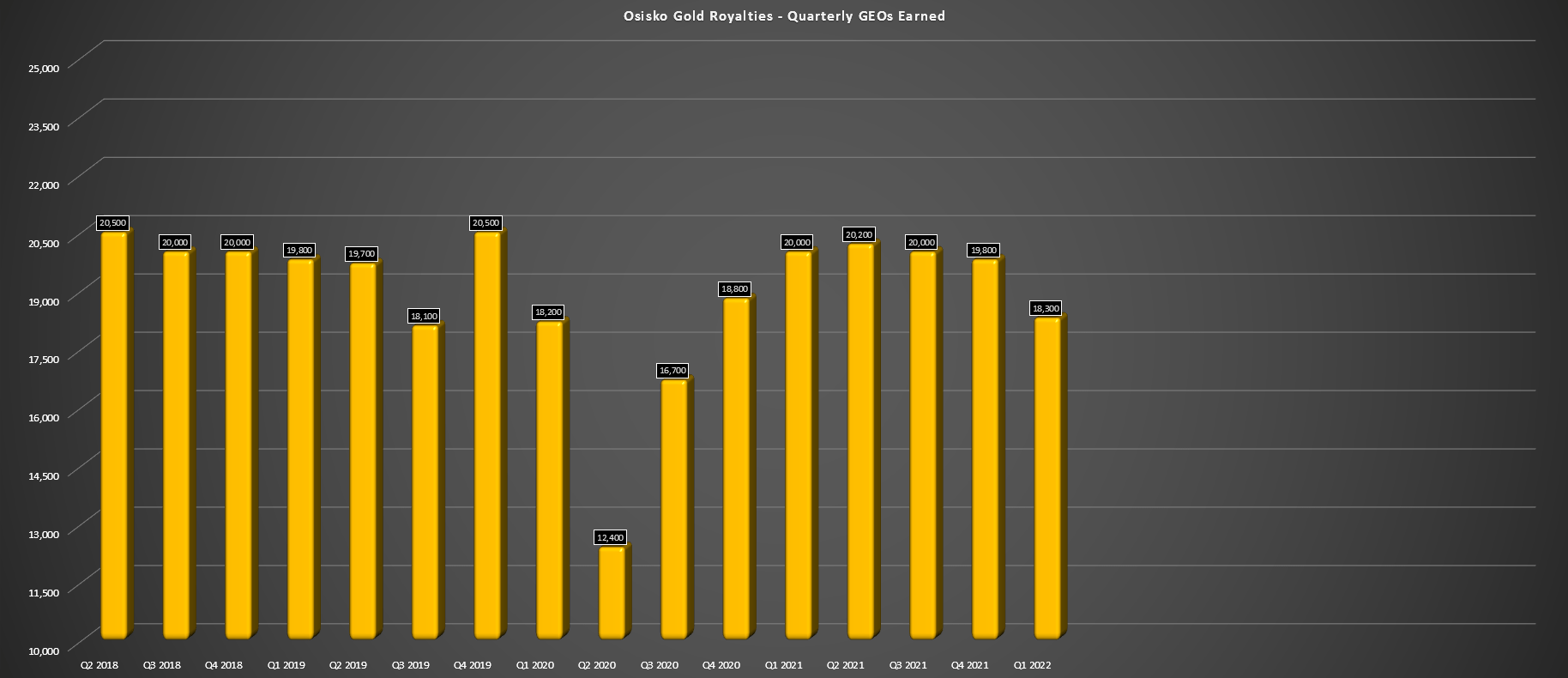
Osisko Gold Royalties Stock Buy The Dips Nyse Or Seeking Alpha

Hydrogen Deuterium Exchange In Mass Spectrometry Kostyukevich 2018 Mass Spectrometry Reviews Wiley Online Library

H2so4 Catalyzed Isobutane Alkylation Under Low Temperatures Promoted By Long Alkyl Chain Surfactant Additives Zheng 2021 Aiche Journal Wiley Online Library

Consequences Of Intrapore Liquids On Reactivity Selectivity And Stability For Aldol Condensation Reactions On Anatase Tio2 Catalysts Kadam 2022 Chemcatchem Wiley Online Library

Effective Factors On Performance Of Zeolite Based Metal Catalysts In Light Hydrocarbon Aromatization